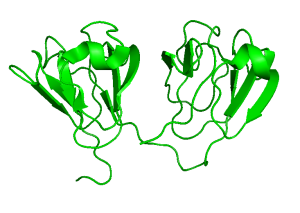
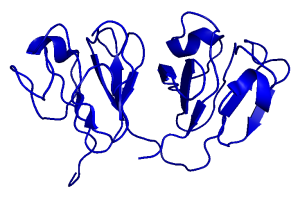
The G18V variant of human γS-crystallin (γS-G18V) is implicated in childhood-onset cataract. We have investigated γS-G18V and its symmetry-related variant γS-G106V, located at the analogous position on the C-terminal domain, and found that these two superficially similar mutations affect stability and solubility quite differently. The solution NMR structures of the wild-type protein (γS-WT) and γS-G18V and their interactions with αB-crystallin reveal that the aggregation mechanism of γS-G18V is related to altered intermolecular interactions of a native-like monomer rather than large-scale misfolding or remodeling of the protein structure. Our results for this system are consistent with a cataractogenesis model where the aggregation of γS-G18V results from depletion of the lens’s finite pool of α-crystallin. Ongoing studies include the investigation of other clinically relevant mutations, as well as those mimicking the effects of age-related cataract.
Wild Type Human Gamma-S Crystallin